OVERVIEW
Tyche® Lumbar Expandable Cage expands when implanted to promote the spine’s natural lordosis. It provides a strong bone-to-cage interface, reducing migration risk.
This one of a kind cage made of lightweight and biocompatible titanium is strong enough to withstand even the most demanding loads on the spine. It is also simple and easy to implant.
FEATURES & BENEFITS
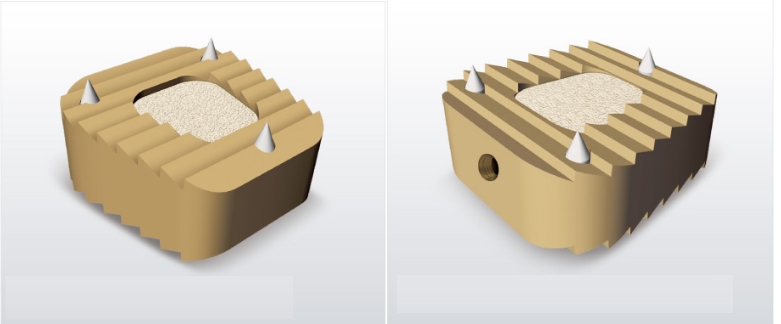
- Cylindrical shape expands in disk space and upper & lower sides become flat.
- External surface ridges stabilize setting and provide a strong bone anchor.
- Expansion creates large inner volume for more bone graft areas.
- Four large openings promote better graft integration.
- The upper and lower parts expose a large area between end-plates and graft.
- The lateral parts enable fusion between implants.
- Four different sizes are available for most clinical requirements.
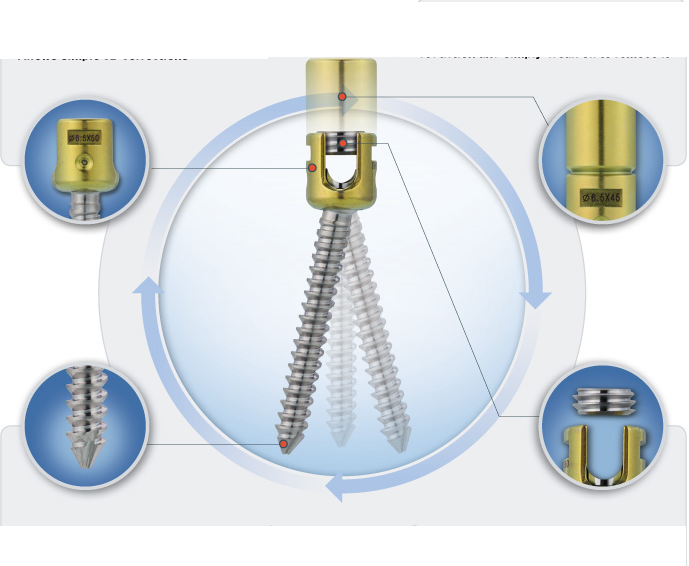
- State-of-the-art instruments accelerate implantation
INDICATION FOR USE
The primary indication for use of the Tyche® intervertebral body fusion device is for painful, degenerative disc disease, persisting for at least 6 months despite intensive conservative care.
A positive concordant discogram is helpful in determining which patients might benefit from an interbody fusion.
INSTRUMENTS
State-of-the-art instruments accelerate implantation









Share Tyche® Lumbar Expandable Cage in Social Media